Curaleaf is the subject of a federal Class Action Lawsuit regarding it’s stock. Their stock went down over 7% after an FDA warning letter was issued to the company regarding CBD marketing. They were making claims on their site that were inconsistent with federal regulations.
Curaleaf is now the defendant of a class-action securities complaint
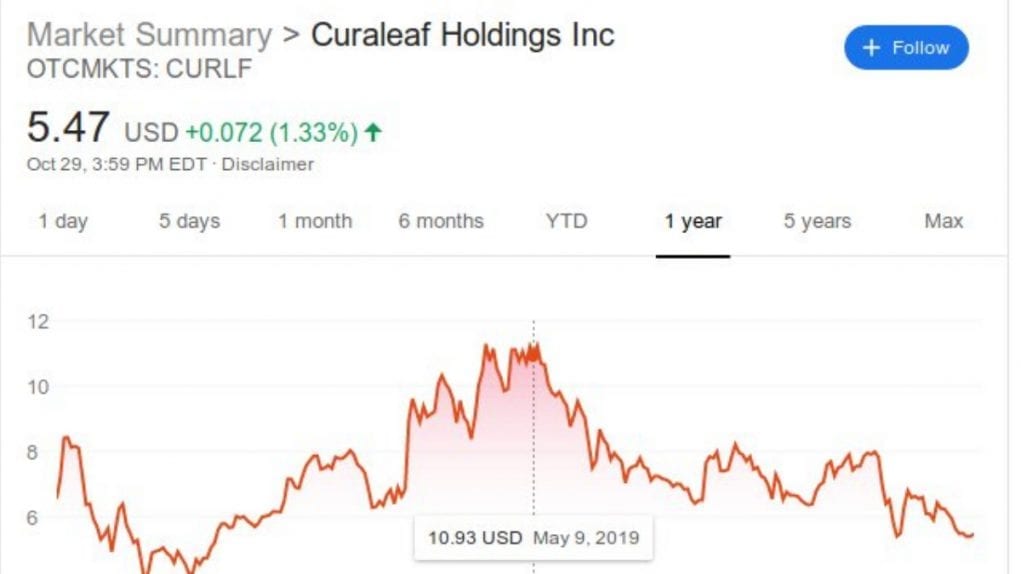
Up until recently, Curaleaf Holdings, Inc. has been one of the largest cannabis companies in the world, after the acquisition of Cura Partners to the tune of nearly $1 billion. When news of that acquisition came out in May of 2019, their stock soared. But the proverbial mighty have fallen since the FDA sent Curaleaf a warning about the claims they were making about their CBD products. Curaleaf pulled all advertising claims about their product’s health benefits.
And then their stock tanked hard, to less than half its May 2019 peak. So now this is what the lawsuit is about, a class-action securities complaint against Curaleaf that was filed on August 5 in the Eastern District of New York. The suit’s complaint is that of violations of the Exchange Act, for federal securities law regarding the making of unfounded claims about a product which causes inflation of the stock price.
Here is RK Attorneys with an explainer video:
For the record: the FDA has approved NO over-the-counter cannabis medications
That is zero, zip, and nada. Not CBD oil, not terpenes, not THC, nothing. They have approved about four medications derived from cannabis products for prescription use only. Here is the FDA clarifying their efforts in cannabis product research, and here is an extensive section of questions and answers by the FDA about cannabis products relative to their medical approval.
FDA quotes:
“To date, the agency has not approved a marketing application for cannabis for the treatment of any disease or condition. FDA has, however, approved one cannabis-derived and three cannabis-related drug products. These approved products are only available with a prescription from a licensed healthcare provider.”
So there it is in black and white. Bought it over the counter? Not approved.
In particular, attention seems to be on Curaleaf for its claims of a veterinary medicine derived from cannabis. From Curaleaf’s former marketing:
“CBD has been shown in initial third-party studies to support a pet’s overall wellness including the potential to help manage pain and anxiety.”
As opposed to that FDA FAQ:
“FDA is aware of some cannabis products being marketed as animal health products. We want to stress that FDA has not approved cannabis for any use in animals, and the agency cannot ensure the safety or effectiveness of these products. For these reasons, FDA cautions pet-owners against the use of such products and recommends that you talk with your veterinarian about appropriate treatment options for your pet.”
Bottom line
There certainly seems substance to the claims of the suit based on these text quotes. We certainly can’t speak for Curaleaf’s motives or intentions, of course. They may forge a new business path and recover yet, who knows?
Share your thoughts on Curaleaf and the FDA here or in our forums. If you think you might be eligible for the lawsuit, email our Assistant Editor Neil Dillard at neil@dabconnection.com.